Dosage forms designed for pediatric use has long been an unmet medical need. The lack of pediatric formulation has led to unsafe behaviors such as breaking adult tablet arbitrarily and dosing randomly for children. Physiologically and pharmaceutically speaking, pediatric medicines are not “mini” versions of adult medicine. Instead, it is necessary to make changes or adjustments in dosage form, dose and administration method. Due to the lack of pediatric dosage forms, there is a pervasive off-label use of adult dosage for pediatric patients, leading to incorrect doses, altered bioavailability, and poor patient compliance, etc. In recent years, the importance of pediatric formulation has been recognized in China. NMPA has issued a set of policies and regulations encouraging the development and innovation in pediatric medicines. Meanwhile, CDE also published a series of technical guidance to better help drug companies in pediatric drug development. In the US, the FDA requires drug companies to submit initial pediatric study plan (iPSP) after Phase II clinical trials. In the EU, the EMA demands drug companies submit pediatric investigation plan (PIP) after Phase I clinical trials and complete the development work in the PIP within certain time limits. Therefore, the demand in pediatric formulation development will continue to increase with time.
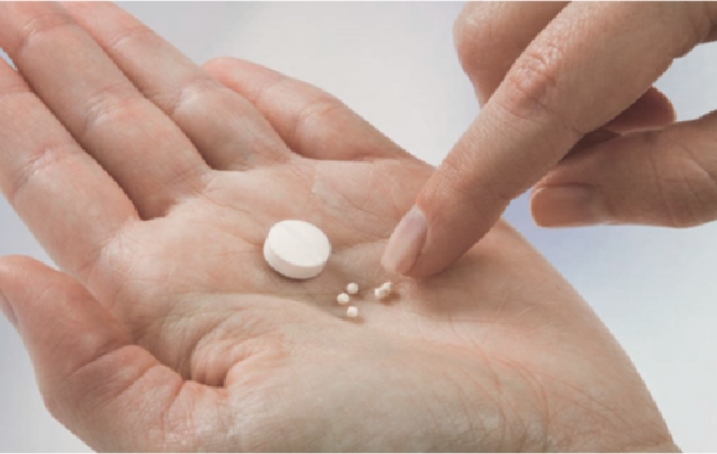
The solution and suspension are typical formulations among various pediatric oral dosage forms, but the longer-term stability, the need for preservatives, and the inaccuracy of dosing, etc. have always been concerns with them. The minitablet based oral granule drug delivery system offers clear advantages. Firstly, it is applicable to the entire pediatric population from neonate to adolescent. There is even a study indicating that a 2 mm minitablet is as acceptable to infants and toddlers as a syrup formulation. Secondly, it has higher flexibility in dose adjustment, greater dosing accuracy, easier swallowability, superior patient compliance, controllable drug release, better stability and convenience in transportation and storage.
There are some technical challenges in pediatric minitablet formulation. For examples, minitablet is small, weighing only a fraction of regular tablet. It requires larger particle sizes for good flowability, and contradictorily, smaller particle sizes for good content uniformity, which must be properly balanced in a formulation. To avoid or minimize attrition and breakage during film coating, it is desirable for minitablet to have good tablet strength. In compression, minitablet needs special, customer-designed tooling and some modifications of the tablet press may be necessary. In film coating, prolonged spray time can often complicate the process. The biggest challenge is minitablet packaging by counting (vs. by volume for regular capsule or sachet/stick-pack) for final presentation in capsule or stick-pack. It requires specialized, customer-designed equipment for accurate and reliably package operation.
Our formulation team are experienced in minitablet technology with deep expertise in key unit operations such as compression, film coating and packaging. We have built the entire process train for minitablet formulation development from preliminary market formulation to final market formulation and to commercialization.
Better acceptability of dose size/volume
Tailored administration method
Accurate dose
Ease and convenience of handling
Acceptable taste, color, mouth feel…
Wide range of doses in clinical study with a single formulation
Easy dose adjustment for different age groups
Amenable to various administration methods
Better stability thus longer shelf-life than liquid dosage
Single formulation for neonate to adolescent possible (minimum SKU, lower cost)
More accessible due to lower cost in manufacturing, shipping and storage