A systematic preformulation study is an important preparation for formulation development. It provides the basis for the compound’s developability assessment, administration route and dosage form selection, formulation composition and process design, and quality control. It is foundational to the development of a safe, efficacious, and quality drug product.
Leveraging the highly respected expertise and capability of Crystal Pharmatech in API form development and solid-state characterization, CFS provides a complete preformulation service to our clients. The services include API form development and solid-state characterization, API physiochemical and biopharmaceutical properties measurement, API stability study, API-excipient compatibility study, and preclinical formulation for animal study, etc. In addition, our Material Science team can conduct various formulation characterization to aid formulation design, process development and trouble shooting. All these help strengthen the protection to our client’s drug products in their life cycle.

Rheometer

Particle Sizer Analyzer

Gas Pycnometer
Crystal Formulation Services boasts a formulation development leadership team with extensive experience and deep expertise in new chemical entity (NCE) drug product development.
Instead of one-size-fit-all platforms or “super-placebo” or quick and rough “fit-for-purpose” formulation approaches in early development, we set right in the beginning the strategy for formulation development specifically for a molecule based on its intended therapeutic area, indication, treatment regimen, available pharmacokinetic and pharmacodynamic data, drug metabolism, molecular profile, API’s physiochemical, biopharmaceutical, stability and mechanical properties.
We then design the formulation composition and process train based on the understanding of material properties of API and excipients such as physical attributes, mechanical properties, functionalities and chemical compatibility. The composition is deliberately designed for the process train in terms of dose range, drug loading, excipient choices and concentrations of every ingredient.
Finally, during the drug product development process we strive to keep a line-of-sight (LOS) of the intended clinical goals, i.e., purpose of the molecule as a medicine, as the driver for all relevant decisions. We perform thorough risk assessment prior to key milestones to guide the design of experiment (DOE) study for the understanding of the impact of selected critical API and excipient properties and composition on product critical quality attribute (CQA), the interaction of composition and process, the impact of critical process parameter (CPP) on CQA. This broad knowledge space (KS) allows an appropriate determination of a reliable design space (DS) and an effective control strategy (CS) in drug product manufacturing.
In another word, abiding by the molecule-material-medicine, dubbed the M3 drug product development philosophy, we design and develop the formulation tailor-made for a molecule to meet its intended clinical needs, with good bioavailability, stability, manufacturability, patient compliance and regulatory acceptance while avoiding significant formulation change from early to late development. As importantly, leveraging the experience and expertise of our technical leadership team, we are able to do all these competitively in terms of cost and timeline.
A drug needs to attain the right exposure in target tissues in order to achieve expected efficacy while avoiding severe adverse effects. The dosage form, delivery route, composition and process should be determined based on the physiochemical and biopharmaceutical properties of the active pharmaceutical ingredient (API). In general, an oral solid dosage ought to be able to disintegrate, dissolve and be absorbed in the gastro-intestine (GI) track. When necessary, the solubility enhancing technology such as amorphous solid dispersion (ASD) may be utilized to increase the bioavailability.
Drug product needs to be stable during the manufacturing and the storage shelf-life and meets the standards of product quality and the requirements for clinical use. Physically, the solid state of API and the disintegration time etc. should maintain their stability; chemically, there should not be any significant degradation, especially, the toxic degradants must be controlled well within acceptable limits; microbiologically, the product package and storage condition should inhibit any growth of microbes. The efficacy and safety of a drug product is ensured only when it is stable physically, chemically and microbiologically.
Drug product quality may be affected by various factors such as batch to batch variability in properties of key ingredients and critical processing parameters, and various major and minor changes during its life cycle. A drug product manufacturing process should be designed based on risk assessment and understanding of critical material attribute (CMA), critical processing parameter (CPP) and their impacts on critical quality attribute (CQA). This should be accompanied by a corresponding control strategy to ensure continued manufacturing of the drug product with high quality.
Molecule
Material


Medicine
Clinical info and needs
DMPK understanding
Pharmacodynamics
Safety info
Molecular profile
API phase
API physiochemical, biopharmaceutical, mechanical properties and stability
Excipient functionality, physical attributes, compatibility
Properties of formulation tailored for process
Label as driver for design
Interaction of composition and process
Impact of API(s) and excipients properties and composition on CQA
Impact of CPP on CQA
Control strategy for drug product


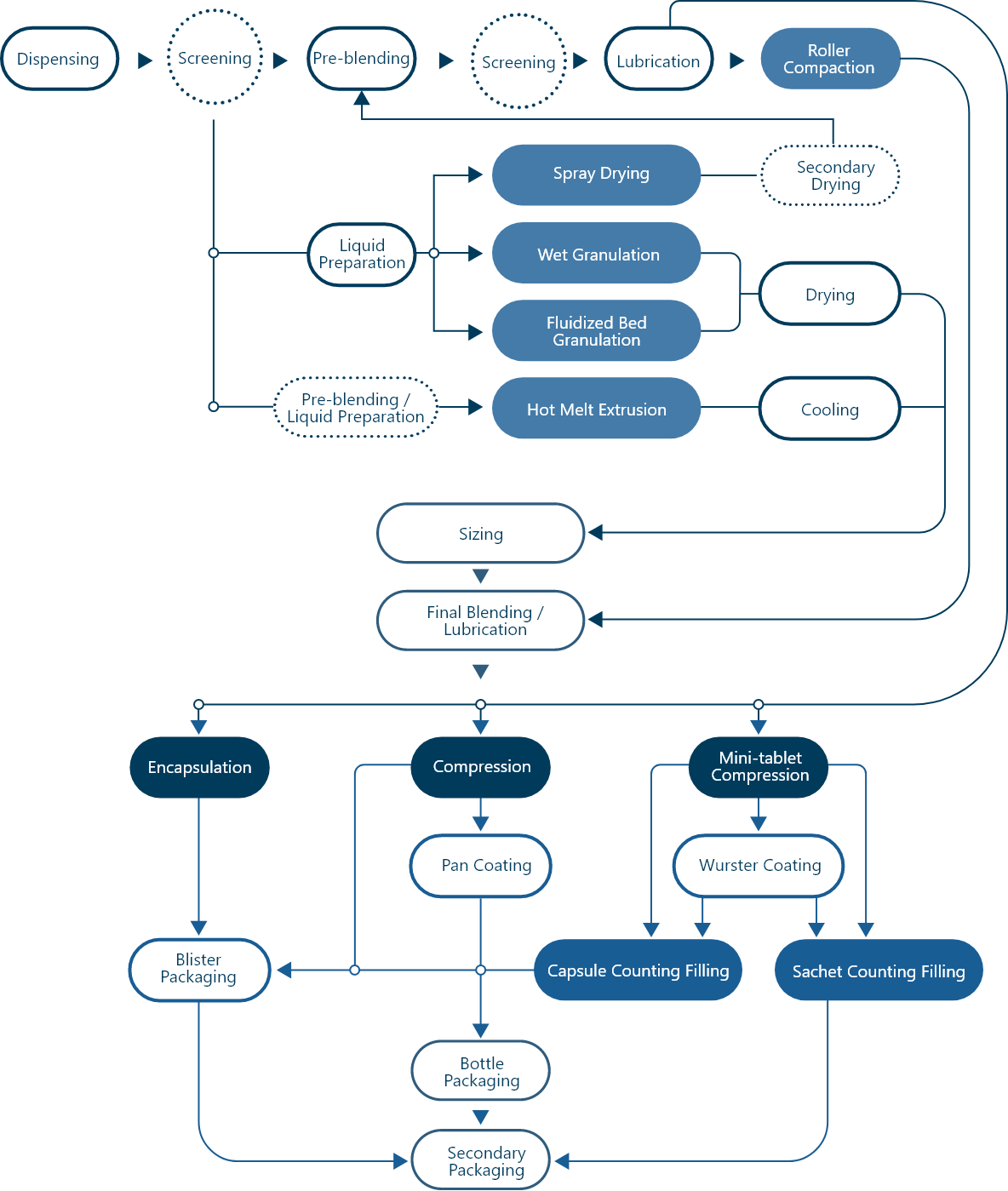
CFS provides full clinical and commercial manufacturing services for OSD:
· Conventional OSD (tablet, capsule, sachet)
· Amorphous Solid Dispersions (ASD) for Insoluble Compounds (Spry Drying Hot Melt Extrusion)
· Pediatric Dosage Form (mini-tab)

Spray Drying

Hot Melt Extrusion

Encapsulation