Most small molecule drugs are formulated as oral solid dosage (OSD) forms, such as tablets or capsules. OSD has been an important part of global pharmaceutical market. With the recent booming of Biotech and new chemical entities (NCE), the demand for CDMO has been increased dramatically. More and more pharma companies focus their resources on discovery and outsource their non-core business, including formulation development and manufacturing to support clinical trials or commercial products. However, oftentimes it is not easy to find a trusted CDMO partner due to the limitation of its technical expertise and service quality. At the same time, there are many challenges across all the formulation development stages for NCEs, for example, limited API supply and tight timeline at early-stage development, and large API requirement for late stage scale up.
To meet the current marketing demand and challenges, CFS has built state-of-art formulation pilot plant and labs, which are equipped with world-class manufacturing equipment and R&D instruments. Our facility provides holistic formulation development capability for different dosage forms. We are striving to provide the best formulation development approach to our clients and become their trusted internal partner.
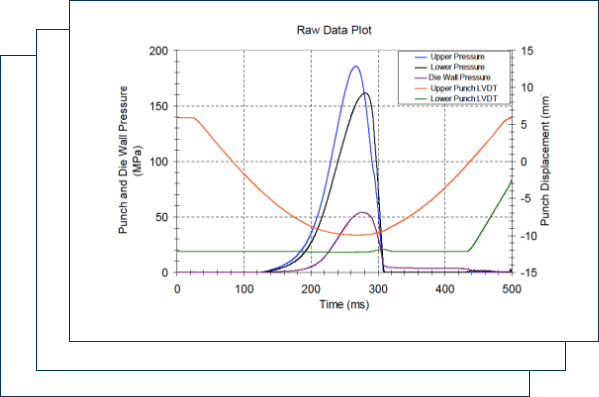
Compaction simulation is one of the core technologies at CFS. It can be used to characterize compaction properties of neat API, excipient, and blends, which will guide formulation development strategy. Specifically, it can simulate the compression process parameters, such as main compression force, precompression force, dwell time, etc., from lab, pilot to commercial scale tableting machines. In addition, it can simulate the roller compaction process, e.g., predict the roll pressure for all kinds of dry granulators. With only a few grams of material, the compaction simulator can predict compression parameters for commercial machines with hundreds of kgs scale. It becomes one of the most efficient and reliable tools for the design and scale up of the dry granulation and tableting process.
Compaction simulation is one of the core technologies at CFS. It can be used to characterize compaction properties of neat API,excipient, and blends, which will guide formulation development strategy.Specifically, it can simulate the compression process parameters, such as maincompression force, precompression force, dwell time, etc., from lab, pilot tocommercial scale tableting machines. In addition, it can simulate the rollercompaction process, e.g., predict the roll pressure for all kinds of drygranulators. With only a few grams of material, the compaction simulator canpredict compression parameters for commercial machines with hundreds of kgsscale. It becomes one of the most efficient and reliable tools for the designand scale up of the dry granulation and tableting process.
· Tablet: Immediate Release (IR), Controlled Release (CR), Bilayer, Mini-tab
· Capsule: Powder, Granules, Multi-particulates, Mini-tab in capsule
· Multi-particulates
· Wet Granulation· Dry Granulation· Blending· Milling
· Compression· Encapsulation· Pan Coating· Fluid Bed Coating

Compaction Simulator

Software Simulation Map