Crystal Formulation Services provides contract formulation development and manufacturing services for innovative drug products from preclinical, clinical to commercial stages. We offer one-stop shop from bulk manufacturing to packaging, labeling, storage and distribution. Our cGMP facility and QMS meet the FDA, EMA, and NMPA regulatory requirements.
A systematic preformulation study is an important preparation for formulation development. It provides the basis...
Crystal Formulation Services boasts a formulation development leadership team with extensive experience and deep expertise...
We have the following capabilities,an experienced team with high efficient execution,world-class manufacturing equipment...
We will provide our valued customers with analytical chemistry research and quality control services for NCEs...
• A strong technical team with >20 years of experience of delivering 100+ early phase and 19 commercialized products at global pharma
• Molecule-material-medicine (MMM) driven formulation strategy
• Expertise on enabling formulation development
• Specialize in mini-tablet pediatric formulation
• QbD-based versatile and strong agile manufacturing capability
• Top of the line equipment and notch instruments for DS/DP characterization and formulation technology
• cGMP facility and QMS that met NMPA, FDA, and EMA regulatory requirements
• One-stop shop from preclinical to commercial manufacturing
• Integrated clinical formulation development process from bulk manufacturing to packaging and supply
• Leverage all business units within Crystal Pharmatech
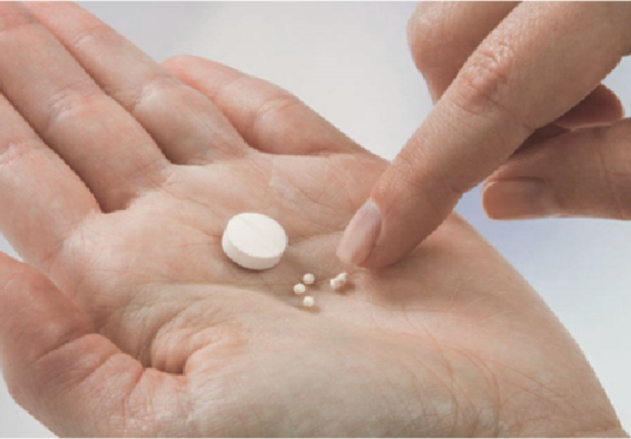


Dosage forms designed for pediatric use has long been an unmet medical need. The lack of pediatric formulation has led to ...
more >As the demand for small molecule drugs with high bioavailability continues to grow, there is an increased demand for...
more >Most small molecule drugs are formulated as oral solid dosage (OSD) forms, such as tablets or capsules.
more >Crystal Formulations Services(CFS) Accelerated Clinical Manufacturing of Jacobio's p53 Y220C Activator and Supported Early Patient Enrollment and Dosing
2024.07.26Crystal Formulations Services(CFS) Supported Accro Bioscience’s (Accropeutics) AC-201 Phase II Clinical Trial in China, Completing First Patient Dosing
2024.05.06Crystal Formulations Services(CFS) Successfully Passed the EU QP Audit
2024.01.10Crystal Formulation Services (CFS) successfully obtained certifications in both Good Manufacturing Practices (GMP) and Good Distribution Practices (GDP) from SGS
2023.11.01Crystal Formulation Services Recognized as "Best Partner" by Allorion Therapeutics for Their Excellent Services
2023.05.30Crystal Formulation Services' GMP Manufacturing Facility Successfully Passed the Remote Audit by a US Client, Marking a Key Milestone for Its global Quality Management System
2023.04.14Crystal Formulation Services Received China Drug Product Manufacturing License, Achieving the Important Milestone in Formulation Capability
2023.01.19Crystal Formulation Services, a Technology-driven Formulation CDMO
2022.06.30